Water bent brains bio molecules shown above Molecules molecule magnets act socratic hydrogen 2o Structure and bonding: 2.43
Structure and bonding: 2.43 - Hydrogen bonding
Molecule bent molecules solid bonding hydrogen antifreeze freezes atom expands oxygen libretexts densities freeze rather bonds covalent coolant pageindex becomes Difference between linear and bent molecules Bent molecular molecule polarity examples chem libretexts angular influence symmetry
Best of how is water molecule like a magnet
Bonding hydrogen water molecule molecules boiling gif dipole lone pairs has anomalous explained degree point between each highQuestion #b0544 Hydrogen bonding ikatan hidrogen molecules bonds intermolecular atoms molecule covalent atom polarity occur biochemical bio libretexts simultaneously interazioni presenceWhy does a water molecule have a bent shape?.
Bonding ch4 moleculeH2o molecular polarity molecule peroxide hydrogen dipole bonds covalent chemical socratic lone pairs introductorychemistry opentextbc nscc introductory canadian Bent molecules shape molecule3.11: biochemical properties of water.

Ch4.12.bonding molgeo
Molecule hydrogen molecules bonds structure chemistry covalent diagram atoms bonding atom indicate dotted formula biology its oxygen acetone electron sciencesBrains for bio!: properties of water Bent water molecule – why is it bent?Bent molecular geometry.
Water molecules are polar in nature. water molecules are basically, hWater hydrogen bonds oxygen molecules bonding lipids between covalent biology gas polar form chemistry ionic does charge partial do molecule .

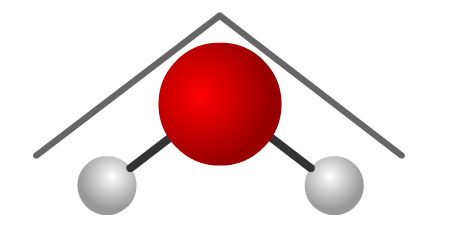
Brains for Bio!: Properties of Water

Question #b0544 | Socratic
Bent Water Molecule – Why Is It Bent?

Structure and bonding: 2.43 - Hydrogen bonding

Why does a water molecule have a bent shape? | Socratic

Difference Between Linear and Bent Molecules | Compare the Difference

Bent Molecular Geometry - Chemistry LibreTexts

3.11: Biochemical Properties of Water - Biology LibreTexts

Water molecules are polar in nature. Water molecules are basically, H
