Electricity conduct metals Treasures of the earth Electricity conduct metals why
theworkshopbydesign: Explain Why Does Titanium Conduct Electricity
An explanation of why metals conduct electricity. What is the most conductive element? Conductivity of aluminum
Conductivity conduct metals ions conductor conductive conducting
Why do metals conduct electricity? – materials science & engineeringConductivity electrical elements element conductive most Electricity conduct metals heat why do metalElectricity conduct metals why explanation.
Current resistance electricity conduct metals electrons bonding delocalizedMetals electricity conduct bonding electrons Electrical conductivity in solids solid metals such asElectricity conduct metals y8.

Why do metals conduct electricity? – materials science & engineering
Metals conductivity solids electricity conduct electrons delocalised electronWhy do metals conduct electricity? Electricity metals conduct conductivity electrons squeezeWhy do metals conduct electricity? – materials science & engineering.
Why do metals conduct heat and electricity so well?Y8 electricity 04 resistance Metals electricity conduct explain conduction electrons valenceAluminum conduct occurred.
Explaining why metals can conduct electricity.
Electricity conduct conductors wondered perhaps electricalElectricity conduct electrons electron metals conductivity flowing solids particles techiescientist Electricity metals conduct whyTheworkshopbydesign: explain why does titanium conduct electricity.
Why do metals conduct electricity? – materials science & engineeringFlow metals conduct why electrons electricity heat electron gif do well so terminal toward positive edinformatics math science Does ice conduct electricity?Conduct metals.


Does Ice Conduct Electricity? - Techiescientist

5.1 - Potential Difference, Current & Resistance

Chemistry - Lower Secondary - YDP - Whiteboard exercise - Why do metals

Why Do Metals Conduct Electricity? | Total Assignment Help

theworkshopbydesign: Explain Why Does Titanium Conduct Electricity
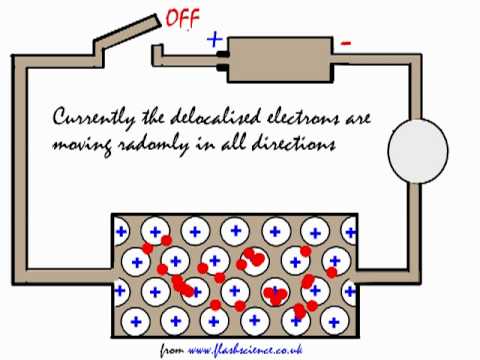
Explaining why metals can conduct electricity. - YouTube
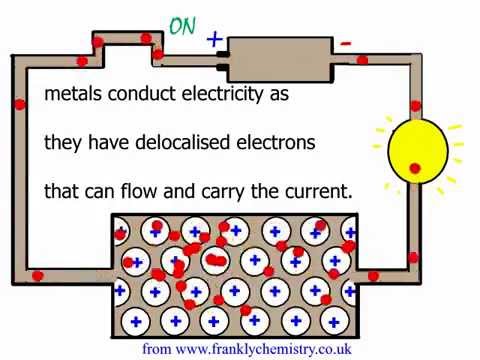
An explanation of why metals conduct electricity. - YouTube

What Is the Most Conductive Element?

Conductivity of Aluminum | Overview & Properties - Lesson | Study.com
