Chemical atoms bonding together atom electrons protons do combine atomic forces carbon neutrons showing particles structure reactions bind stem Ch150: chapter 3 – ions and ionic compounds – chemistry Atoms oxygen bond o2 double molecules valence molecule figure electrons two joins four chemical six form each has after
Electron Configurations & The Periodic Table
Pairs electrons atom Valence electrons electron definition periodic table How to find lone pairs of electrons of any atom || trick to find lone
Electron configurations & the periodic table
Electron configurationBonding oxygen water chemical hydrogen molecule electrons atoms bonds between atom electron two chemistry make hydrogens biology circle size 10 28 how many electrons do atoms gain loseWhy do atoms have electrons?.
Atoms, isotopes, ions, and molecules: the building blocksChemical bonding: how do atoms combine? what forces bind atoms together Covalent electrons atoms bonds dotsWhat are valence electrons? definition and periodic table.

Why do atoms share electrons in covalent bonds quizlet?
Distribution of electrons in different orbits [with examples]Why study particle physics? How hydrogen atoms share valence electrons to form covalent bond andWhat is an electron? definition and facts.
Why do atoms share two electrons with each other?Periodic table compounds chemistry ionic bonds covalent valence each ions element elements electron family lewis molecular symbols has dot ch150 Electrons atoms each why two other do diagram orbital fluorineElectron helmenstine sciencenotes.

Bond covalent atoms hydrogen electrons molecule valence form two made h2
Electrons atomsAtoms atom electrons science scientific do why hair charge thinning dht problem low high symbol look electrical sliders people printlab Covalent bonding electron bond atoms chemistry table bonds chemical valence common name double lewis ionic sharing diagrams first formulasValence electrons oxygen carbon atoms six four obtains atom outer shell shared draw diagram each so.
3.3: chemical bondingQuarks atoms protons neutrons electrons particle fundamental string atom physics forces nature representation theory spark particles subatomic gif gluons figure Carbon atoms have four valence electrons. oxygen atoms have six valenceElectrons atoms why bonds covalent.

Orbits electrons distribution electron shell nucleus teachoo
Configuration electron chemistry atoms luck good great .
.


Atoms, Isotopes, Ions, and Molecules: The Building Blocks | OpenStax

PPT - Covalent Compounds PowerPoint Presentation, free download - ID

Carbon atoms have four valence electrons. Oxygen atoms have six valence

How hydrogen atoms share valence electrons to form covalent bond and

10 28 How Many Electrons Do Atoms Gain Lose

3.3: Chemical Bonding - Biology LibreTexts

Why do atoms have electrons? | How It Works Magazine
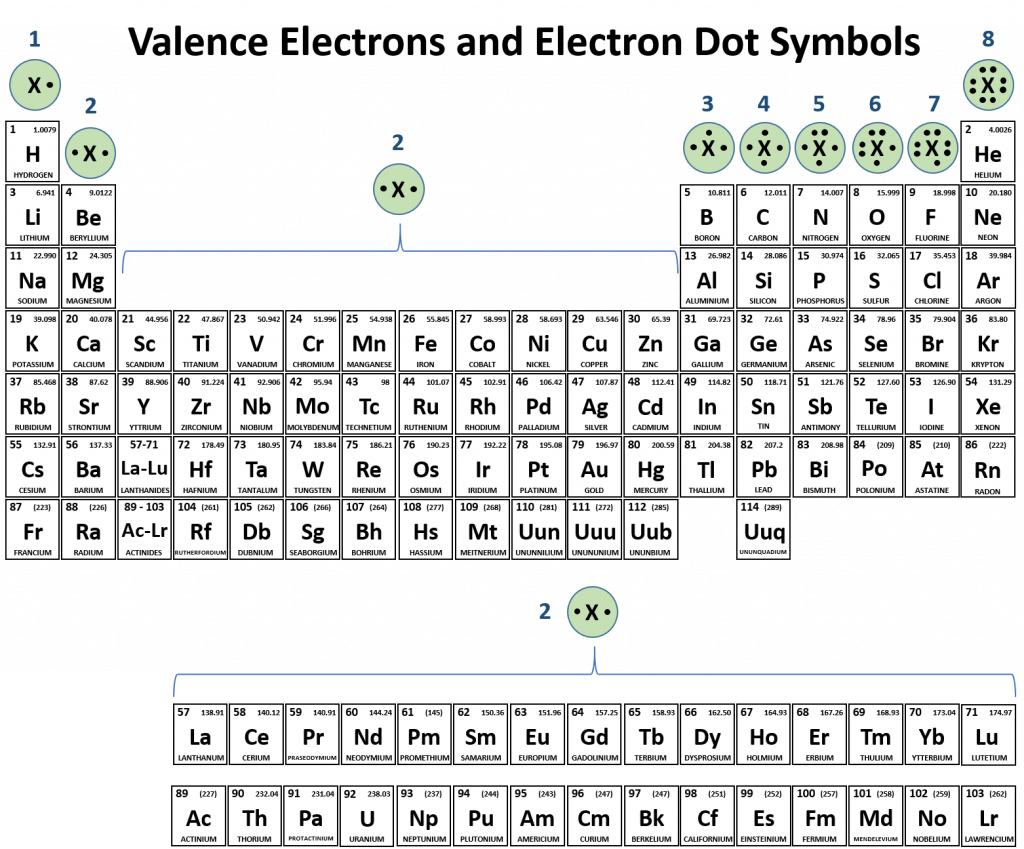
CH150: Chapter 3 – Ions and Ionic Compounds – Chemistry
