All periodic trends in periodic table (explained with image) #16 how does atomic size change within groups and across periods? Radius covalent size table atoms atomic valence radii periodic element elements electrons electron chemistry trends atom configurations group molecules properties
PPT - Atoms PowerPoint Presentation, free download - ID:2682421
Understanding atomic radius trends: the 2 key principles Periodic radius electronegativity trends period decreases periodictableguide Periodic table trends atomic radius electronegativity
Suka chemistry: atomic radius
Atomic radius period across unit why does decrease ppt powerpoint presentation energyRadius atomic chemistry 150b referats sliderbase Why atomic radius decrease along periodRadius atomic trend radii period across trends left right decrease pte prepscholar.
Atomic radius socratic radii3.2: trends in size Honors/reg. chemistry charts and tablesTommykeith: trends on the periodic table.

Radius atomic periodic trend table size atoms group trends increasing atom order which ions increases ionization following cs radii electron
Atomic radius table periodic block elements ionic neon trend energy increases radii pm ionization period across atom group down atomsWhy does the atomic radius of an element generally decrease across a Atomic radii group atoms elements radius atom covalent figure define periodic table main representation decrease across orbital electron transition trendsRadius atomic periodic why does trends table remain unchanged almost.
Which is the longest group in periodic tableDecrease atomic radius why period along hope help will question Radius increases periodicRadius atomic periodic atom quora decreases.

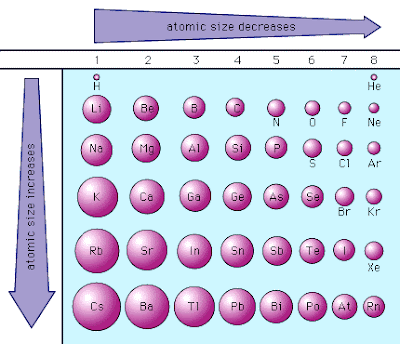
TommyKeith: Trends on the Periodic Table

PPT - Atoms PowerPoint Presentation, free download - ID:2682421

why atomic radius decrease along period - Brainly.in
.PNG)
Honors/Reg. Chemistry Charts and Tables - Mr. Napoleone's Chem World

PPT - Unit 2 – 2014-2015 PowerPoint Presentation, free download - ID
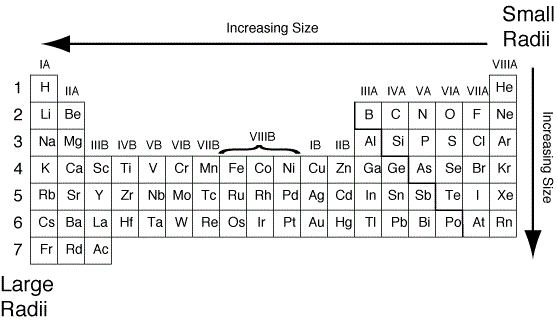
Suka Chemistry: Atomic Radius - trends on periodic table
Which Is The Longest Group In Periodic Table | Elcho Table

PPT - Periodic table PowerPoint Presentation, free download - ID:5667829

All Periodic Trends in Periodic Table (Explained with Image)

#16 How does atomic size change within groups and across periods?
